Learning Outcomes
i. Comprehend the Lewis theory of acids and bases.
ii. Identify Lewis acids and bases based on their electron-pair donating or accepting abilities.
iii. Understand the concept of a Lewis adduct.
iv. Distinguish between Lewis and Bronsted-Lowry acid-base theories.
Introduction
In the previous lessons, we explored the Arrhenius and Bronsted-Lowry theories of acids and bases, which focus on proton transfer as the defining characteristic of these substances. In this lesson, we delve into the Lewis theory of acids and bases, an alternative approach that expands the scope of acid-base chemistry beyond the limitations of proton transfer.
i. The Essence of Lewis Acid-Base Theory
G.N. Lewis proposed his concept of acids and bases in 1923, independent of the Bronsted-Lowry theory. According to the Lewis theory, an acid is a substance that can accept a pair of electrons to form a covalent bond, while a base is a substance that can donate a pair of electrons to form a covalent bond.
ii. Lewis Adducts: The Products of Electron-Pair Interactions
When a Lewis acid and a Lewis base interact, they form a complex called a Lewis adduct. The Lewis acid accepts an electron pair from the Lewis base, resulting in the formation of a covalent bond between them.
Examples of Lewis Acids and Bases
Examples of Lewis acids include:
- Boron trifluoride (BF3)
- Aluminum chloride (AlCl3)
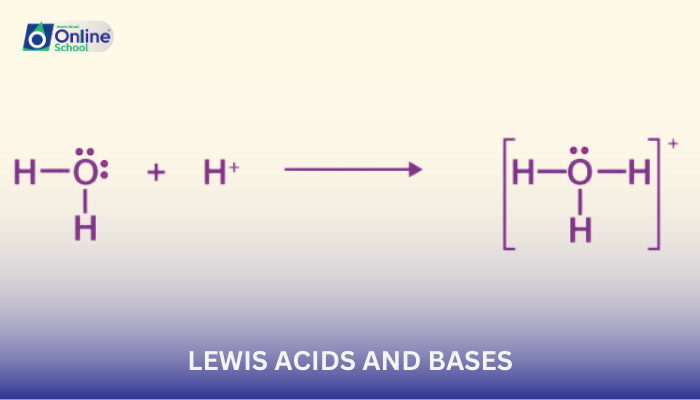
- Hydrogen ions (H+)
- Metal cations (e.g., Fe3+, Cu2+)
Examples of Lewis bases include:
- Ammonia (NH3)
- Water (H2O)
- Hydroxide ions (OH-)
- Anions (e.g., Cl-, F-, I-)
iii. Distinguishing Lewis from Bronsted-Lowry Acid-Base Theories
While the Lewis and Bronsted-Lowry theories share some similarities, they differ in their scope and applicability. The Bronsted-Lowry theory focuses exclusively on proton transfer, while the Lewis theory encompasses a broader range of electron-pair interactions.
The Lewis theory of acids and bases provides a complementary perspective on acid-base chemistry, expanding our understanding of these substances beyond the limitations of proton transfer. By recognizing the concept of electron-pair donation and acceptance, we gain insights into the formation of Lewis adducts and the behavior of acids and bases in various chemical environments. This knowledge has far-reaching applications in various fields, including organic chemistry, inorganic chemistry, and materials science.